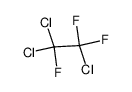
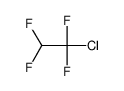
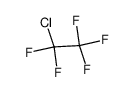
| Product name | 1,1,2-Trichlorotrifluoroethane |
|---|
| Product number | - |
|---|---|
| Other names | Ethane, 1,1,2-trichloro-1,2,2-trifluoro- |
| Identified uses | For industry use only. Food Additives: EXTRACTION_SOLVENT |
|---|---|
| Uses advised against | no data available |
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
Hazardous to the ozone layer, Category 1
Eye irritation, Category 2
Hazardous to the aquatic environment, long-term (Chronic) - Category Chronic 2
2.2 GHS label elements, including precautionary statements| Pictogram(s) |   |
|---|---|
| Signal word | Warning |
| Hazard statement(s) | H319 Causes serious eye irritation H411 Toxic to aquatic life with long lasting effects |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P280 Wear protective gloves/protective clothing/eye protection/face protection. P273 Avoid release to the environment. |
| Response | P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337+P313 If eye irritation persists: Get medical advice/attention. P391 Collect spillage. |
| Storage | none |
| Disposal | P502 Refer to manufacturer or supplier for information on recovery or recycling P501 Dispose of contents/container to ... |
none
3.Composition/information on ingredients 3.1 Substances| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| 1,1,2-Trichlorotrifluoroethane | 1,1,2-Trichlorotrifluoroethane | 76-13-1 | none | 100% |
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaledFresh air, rest. Artificial respiration may be needed. Refer for medical attention.
In case of skin contactRemove contaminated clothes. Rinse skin with plenty of water or shower. Refer for medical attention .
In case of eye contactFirst rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowedRinse mouth. Refer for medical attention .
4.2 Most important symptoms/effects, acute and delayedInhalation causes irritation of the nose, throat, and lungs. High concentrations may cause death by respiratory failure or asphyxiation. May produce superficial skin burns or defatting type dermatitis and may irritate the eyes. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessaryVictims of freon inhalation require management for hypoxic, CNS anesthetic, & cardiac symptoms. Patients must be removed from the exposure environment, & high flow supplemental oxygen should be utilized. The respiratory system should be evaluated for injury, aspiration, or pulmonary edema & treated appropriately. CNS findings should be treated supportively. A calm environment with no physical exertion is imperative to avoid increasing endogenous adrenegic levels. Exogenous adrenergic drugs must not be used to avoid inducing sensitized myocardial dysrhythmias. Atropine is ineffective in treating bradyarrhythmias. For ventricular dysrhythmias, diphenylhydantoin & countershock may be effective. Cryogenic dermal injuries should be treated by water bath rewarming at 40-42°C until vasodilatory flush has returned. Elevation of the limb & standard frostbite management with late surgical debridement should be utilized. Ocular exposure requires irrigation & slit lamp evaluation for injury. /Freons/
5.Fire-fighting measures 5.1 Extinguishing media Suitable extinguishing mediaFirefighters should wear self-contained, NIOSH-approved breathing apparatus for protection against suffocation and possible toxic decomposition products. Proper eye and skin protection should be provided. Use water spray to keep fire-exposed containers cool and to knock down vapors which may result from product decomposition.
5.2 Specific hazards arising from the chemicalSpecial Hazards of Combustion Products: Toxic gases including hydrogen chloride, hydrogen fluoride, and very small amounts of phosgene, fluorine and chlorine are produced. Behavior in Fire: While no flash point is reported, the material may burn if ignited by a high intensity heat source. (USCG, 1999)
5.3 Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures 6.1 Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautionsPersonal protection: self-contained breathing apparatus. Do NOT let this chemical enter the environment. Collect leaking and spilled liquid in sealable containers as far as possible. Absorb remaining liquid in sand or inert absorbent. Then store and dispose of according to local regulations.
6.3 Methods and materials for containment and cleaning upAlways wear recommended personal protective equipment. Immediately evacuate the area and provide maximum ventilation. Try to eliminate all ignition sources. Unprotected personnel should move upwind from spill. Only personnel equipped with proper respiratory and eye/skin protection should be permitted in the area. Dike area to contain the spill. Take precautions as necessary to prevent contamination of ground and surface waters. For large spills, pump material into appropriate containers. For small spills, recover or absorb spilled material using an absorbent designed for chemical spills such as Hazsorb pillows. Place used absorbents into closed DOT approved containers for disposal. After all visible traces have been removed, thoroughly wet vacuum the area. DO NOT flush into sewer. If the area of the spill is porous, removal of contaminated earth/surface may be required.
7.Handling and storage 7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilitiesSeparated from metals and alloys. See Chemical Dangers. Cool. Ventilation along the floor.Keep container closed when not in use. DO NOT store in open, unlabeled or mislabeled containers. Store in a cool, well- ventilated area of low fire risk. Protect container and its fittings from physical damage. Storage in subsurface locations should be avoided. Close valve tightly after use and when empty. If container temperature exceeds boiling point, cool the container before opening.
8.Exposure controls/personal protection 8.1 Control parameters Occupational Exposure limit valuesRecommended Exposure Limit: 10 Hour Time-Weighted Average: 1,000 ppm (7,600 mg/cu m).
Recommended Exposure Limit: 15 Minute Short-Term Exposure Limit: 1,250 ppm (9,500 mg/cu m).
Biological limit valuesno data available
8.2 Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE) Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protectionWear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protectionWear dust mask when handling large quantities.
Thermal hazardsno data available
9.Physical and chemical properties| Physical state | Colorless liquid with a sweet, ether-like odor |
|---|---|
| Colour | Liquid |
| Odour | Nearly odorless |
| Melting point/ freezing point | -36.4ºC |
| Boiling point or initial boiling point and boiling range | 47.6ºC |
| Flammability | Noncombustible Liquid at ordinary temperatures, but the gas will ignite and burn weakly at 680°C.Combustible under specific conditions. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | 195ºC |
| Auto-ignition temperature | 680°C |
| Decomposition temperature | no data available |
| pH | no data available |
| Kinematic viscosity | 0.497 mPa.s at 48.9°C (liquid); 0.0108 mPa.s at 49°C (gas) |
| Solubility | In water:0.02 g/100 mL. Slightly soluble |
| Partition coefficient n-octanol/water (log value) | log Kow = 3.16 |
| Vapour pressure | 5.5 psi ( 20 °C) |
| Density and/or relative density | 1.575 |
| Relative vapour density | 6.5 (vs air) |
| Particle characteristics | no data available |
no data available
10.2 Chemical stabilityStable under recommended storage conditions.
10.3 Possibility of hazardous reactionsNot combustible.The vapour is heavier than air and may accumulate in lowered spaces causing a deficiency of oxygen.1,1,2-TRICHLORO-1,2,2-TRIFLUOROETHANE yields violent reactions with Al, Ba, Li, Sm, Na/K alloy and Ti . May react exothermically with aluminum.
10.4 Conditions to avoidno data available
10.5 Incompatible materialsMixtures /of aluminum/ with fluorotrichloroethane and with trichlorotrifluoroethane will flash or spark on heavy impact.
10.6 Hazardous decomposition productsDecomposes on contact with hot surfaces or flames. This produces toxic and corrosive gases of hydrogen chloride, phosgene, hydrogen fluoride and carbonyl fluoride. Reacts violently with powdered metals. This generates fire and explosion hazard. Attacks magnesium and its alloys.
11.Toxicological information Acute toxicity- Oral: LD50 Rat oral 43 g/kg
- Inhalation: no data available
- Dermal: no data available
no data available
Serious eye damage/irritationno data available
Respiratory or skin sensitizationno data available
Germ cell mutagenicityno data available
CarcinogenicityA4: Not classifiable as a human carcinogen.
Reproductive toxicityno data available
STOT-single exposureno data available
STOT-repeated exposureno data available
Aspiration hazardno data available
12.Ecological information 12.1 Toxicity- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
AEROBIC: 1,1,2-Trichloro-1,2,2-trifluoroethane, present at 100 mg/L, reached 0-5% of its theoretical BOD in 4 weeks using an activated sludge inoculum at 30 mg/L and the Japanese MITI test(1).
12.3 Bioaccumulative potentialBCFs of 11-33 and 14-86 were measured for 1,1,2-trichloro-1,2,2-trifluoroethane at concentrations of 0.198 and 0.0198 mg/L, respectively(1). According to a classification scheme(2), these BCF values suggest bioconcentration in aquatic organisms is low to moderate.
12.4 Mobility in soilThe Koc of 1,1,2-trichloro-1,2,2-trifluoroethane is estimated as 552(SRC), using a log Kow of 3.16(1) and a regression-derived equation(2). According to a classification scheme(3), this estimated Koc value suggests that 1,1,2-trichloro-1,2,2-trifluoroethane is expected to have low mobility in soil.
12.5 Other adverse effectsno data available
13.Disposal considerations 13.1 Disposal methods ProductThe material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information 14.1 UN Number| ADR/RID: UN1078 | IMDG: UN1078 | IATA: UN1078 |
| ADR/RID: REFRIGERANT GAS, N.O.S. |
| IMDG: REFRIGERANT GAS, N.O.S. |
| IATA: REFRIGERANT GAS, N.O.S. |
| ADR/RID: 9 | IMDG: 9 | IATA: 9 |
| ADR/RID: III | IMDG: III | IATA: III |
| ADR/RID: yes | IMDG: yes | IATA: yes |
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Codeno data available
15.Regulatory information 15.1 Safety, health and environmental regulations specific for the product in question| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| 1,1,2-Trichlorotrifluoroethane | 1,1,2-Trichlorotrifluoroethane | 76-13-1 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
| Creation Date | Aug 17, 2017 |
|---|---|
| Revision Date | Aug 17, 2017 |
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/