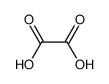
| Product name | oxalic acid |
|---|
| Product number | - |
|---|---|
| Other names | Aktisal |
| Identified uses | For industry use only. Paint additives and coating additives not described by other categories,Photosensitive chemicals,Surface active agents |
|---|---|
| Uses advised against | no data available |
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
Acute toxicity - Oral, Category 4
Acute toxicity - Dermal, Category 4
2.2 GHS label elements, including precautionary statements| Pictogram(s) |  |
|---|---|
| Signal word | Warning |
| Hazard statement(s) | H302 Harmful if swallowed H312 Harmful in contact with skin |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. |
| Response | P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor/…if you feel unwell. P330 Rinse mouth. P302+P352 IF ON SKIN: Wash with plenty of water/... P312 Call a POISON CENTER/doctor/…if you feel unwell. P321 Specific treatment (see ... on this label). P362+P364 Take off contaminated clothing and wash it before reuse. |
| Storage | none |
| Disposal | P501 Dispose of contents/container to ... |
none
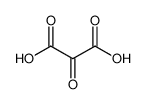
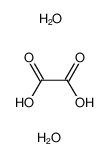
3.Composition/information on ingredients 3.1 Substances| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| oxalic acid | oxalic acid | 144-62-7 | none | 100% |
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaledFresh air, rest. Half-upright position. Refer immediately for medical attention.
In case of skin contactRemove contaminated clothes. Rinse skin with plenty of water or shower for at least 15 minutes. Refer for medical attention .
In case of eye contactRinse with plenty of water (remove contact lenses if easily possible). Refer immediately for medical attention.
If swallowedRinse mouth. Do NOT induce vomiting. Refer immediately for medical attention.
4.2 Most important symptoms/effects, acute and delayedAs dust or as a solution, can cause severe burns of eyes, skin, or mucous membranes. Ingestion of 5 grams has caused death with symptoms of nausea, shock, collapse, and convulsions coming on rapidly. Repeated or prolonged skin exposure can cause dermatitis and slow-healing ulcers. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessaryTreatment should be rapidly instituted by giving a dilute solution of calcium lactate, lime water, finely pulverized chalk, plaster, and/or milk to supply large amounts of calcium to inactivate oxalate by forming an insoluble calcium salt in the stomach. Gastric lavage is controversial, since this may compound an already severe corrosive lesion in the esophagus or stomach. However, if used, gastric lavage should be done with limewater (calcium hydroxide). Intravenous gluconate or calcium chloride solutions should be given to prevent hypocalcemic tetany; in severe cases parathyroid extract also has been given. ... Additionally, acute renal failure should be anticipated, and careful fluid management is necessary. /Oxalates/
5.Fire-fighting measures 5.1 Extinguishing media Suitable extinguishing mediaUSE WATER SPRAY, DRY CHEM, "ALC RESISTANT" FOAM, OR CARBON DIOXIDE. DUST MAY BE REDUCED WITH WATER SPRAY. AQUEOUS SOLUTION MUST BE CONTAINED FOR DISPOSAL. USE WATER TO KEEP FIRE-EXPOSED CONTAINERS COOL. WATER MAY CAUSE FOAMING OF MOLTEN MATERIAL. /OXALIC ACID DIHYDRATE/
5.2 Specific hazards arising from the chemicalSpecial Hazards of Combustion Products: Generates poisonous gases (USCG, 1999)
5.3 Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures 6.1 Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautionsPersonal protection: particulate filter respirator adapted to the airborne concentration of the substance, protective gloves and safety goggles. Sweep spilled substance into covered plastic containers. If appropriate, moisten first to prevent dusting. Wash away remainder with plenty of water.
6.3 Methods and materials for containment and cleaning upCover with soda ash or sodium bicarbonate. Mix and add water. Neutralize and drain into a drain with sufficient water.
7.Handling and storage 7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilitiesSeparated from strong oxidants and food and feedstuffs. Dry. Well closed.STORE IN COOL, DRY, WELL-VENTILATED LOCATION. /OXALIC ACID DIHYDRATE/
8.Exposure controls/personal protection 8.1 Control parameters Occupational Exposure limit valuesRecommended Exposure Limit: 10 Hr Time-Weighted Avg: 1 mg/cu m.
Recommended Exposure Limit: 15 Min Short-Term Exposure Limit: 2 mg/cu m.
Biological limit valuesno data available
8.2 Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE) Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protectionWear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protectionWear dust mask when handling large quantities.
Thermal hazardsno data available
9.Physical and chemical properties| Physical state | Odorless white solid |
|---|---|
| Colour | ANHYDROUS OXALIC ACID, CRYSTALLIZED FROM GLACIAL ACETIC ACID IS ORTHORHOMBIC, CRYSTALS BEING PYRAMIDAL OR ELONGATED OCTAHEDRA |
| Odour | Odorless. |
| Melting point/ freezing point | 189-191ºC |
| Boiling point or initial boiling point and boiling range | Sublimes (NIOSH, 2016) |
| Flammability | Combustible SolidCombustible. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | 101-157ºC |
| Auto-ignition temperature | Not flammable (USCG, 1999) |
| Decomposition temperature | no data available |
| pH | no data available |
| Kinematic viscosity | no data available |
| Solubility | In water:90 g/L (20 ºC) |
| Partition coefficient n-octanol/water (log value) | -0.81 |
| Vapour pressure | <0.01 mm Hg ( 20 °C) |
| Density and/or relative density | 1.9 |
| Relative vapour density | 4.4 (vs air) |
| Particle characteristics | no data available |
no data available
10.2 Chemical stabilityOXALIC ACID CAN BE DEHYDRATED BY CAREFUL DRYING @ 100 DEG C, BUT LOSSES OCCUR THROUGH SUBLIMATION /OXALIC ACID DIHYDRATE/
10.3 Possibility of hazardous reactionsOXALIC ACID is hygroscopic and sensitive to heat. This compound may react violently with furfuryl alcohol, silver, sodium, perchlorate, sodium hypochlorite, strong oxidizers, sodium chlorite, acid chlorides, metals and alkali metals. . The heating of mixtures of Oxalic acid and urea has lead to explosions. This is due to the rapid generation of the gases CO2, CO, and NH3 [Praxis Naturwiss. Chem., 1987, 36(8), 41-42]. Oxalic acid and urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gases, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42].
10.4 Conditions to avoidno data available
10.5 Incompatible materialsReacts with strong alkalies, strong oxidizing materials, chlorites, and hypochlorites. /Oxalic acid dihydrate/
10.6 Hazardous decomposition products... DECOMP PRODUCTS INCL CARBON MONOXIDE & FORMIC ACID.
11.Toxicological information Acute toxicity- Oral: LDLo Dog oral 1000 mg/kg
- Inhalation: no data available
- Dermal: no data available
no data available
Serious eye damage/irritationno data available
Respiratory or skin sensitizationno data available
Germ cell mutagenicityno data available
Carcinogenicityno data available
Reproductive toxicityno data available
STOT-single exposureno data available
STOT-repeated exposureno data available
Aspiration hazardno data available
12.Ecological information 12.1 Toxicity- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
Six tests at oxalic acid initial concns of 3.3 to 10 ppm exhibited 75 to 202 %BODT over an incubation period of 5 days in an aerobic screening study using sewage inoculum(1). A 78 and 55.5 %BODT for oxalic acid was measured under aerobic conditions over a period of 5 days in screening tests at 20°C using sewage inoculum(2). Oxalic acid at initial concns of 0.00375, 0.0375, and 0.375 ppm exhibited 95, 99, and 100% degradation, respectively, in an aerobic screening study at 25°C using sewage inoculum(3). In another screening study using sewage inoculum, 68 and 64 %BODT were measured for oxalic acid at initial concns of 10 and 20 ppm, respectively, over a 5 day incubation period(4). An 89 %BODT was measured for oxalic acid (10 ppm initial concn) in an aerobic screening study using sewage inoculum at 19.5-20.5°C over an incubation period of 5 days(5).
12.3 Bioaccumulative potentialBased on an average experimental water solubility of 220,000 mg/L at 25°C(1) and a regression derived equation(2), the BCF for oxalic acid can be estimated to be approximately 0.6(SRC) and therefore should not be expected to bioconcentrate in aquatic organisms(SRC).
12.4 Mobility in soilBased on an average experimental water solubility of 220,000 mg/L at 25°C(1) and a regression derived equation(2), the Koc for undissociated oxalic acid can be estimated to be approximately 5. This Koc value indicates that oxalic acid will have very high mobility in soil(3); therefore, adsorption to soil and sediment may not be an important fate process. Based on pKa1 and pKa2 values of 1.25 and 4.28(4) respectively, oxalic acid will exist primarily as the oxalate ion under environmental conditions (pH 5-9). No experimental data are available to determine whether the oxalate ion will adsorb to sediment or soil more strongly than its estimated Koc value indicates(SRC).
12.5 Other adverse effectsno data available
13.Disposal considerations 13.1 Disposal methods ProductThe material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information 14.1 UN Number| ADR/RID: Not dangerous goods. | IMDG: Not dangerous goods. | IATA: Not dangerous goods. |
| ADR/RID: unknown |
| IMDG: unknown |
| IATA: unknown |
| ADR/RID: Not dangerous goods. | IMDG: Not dangerous goods. | IATA: Not dangerous goods. |
| ADR/RID: Not dangerous goods. | IMDG: Not dangerous goods. | IATA: Not dangerous goods. |
| ADR/RID: no | IMDG: no | IATA: no |
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Codeno data available
15.Regulatory information 15.1 Safety, health and environmental regulations specific for the product in question| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| oxalic acid | oxalic acid | 144-62-7 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Not Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
| Creation Date | Aug 12, 2017 |
|---|---|
| Revision Date | Aug 12, 2017 |
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/