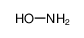| Product name | hydroxylamine |
|---|
| Product number | - |
|---|---|
| Other names | HYDROXYLAMINE |
| Identified uses | For industry use only. Intermediates |
|---|---|
| Uses advised against | no data available |
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
no data available
2.2 GHS label elements, including precautionary statements| Pictogram(s) | no data available |
|---|---|
| Signal word | no data available |
| Hazard statement(s) | no data available |
| Precautionary statement(s) | |
| Prevention | no data available |
| Response | no data available |
| Storage | no data available |
| Disposal | no data available |
no data available
3.Composition/information on ingredients 3.1 Substances| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| hydroxylamine | hydroxylamine | 7803-49-8 | none | 100% |
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaledFresh air, rest. Refer for medical attention. See Notes.
In case of skin contactRemove contaminated clothes. Rinse and then wash skin with water and soap. Refer for medical attention .
In case of eye contactFirst rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowedRinse mouth. Refer for medical attention . See Notes.
4.2 Most important symptoms/effects, acute and delayedINHALATION: Moderately toxic by inhalation and oral routes with the following symptoms possible: headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported. EYES: Corrosive - highly irritating. SKIN: Irritating or corrosive to skin. INGESTION: Moderately toxic by inhalation and oral routes with the following symptoms possible; headache, vertigo, tinnitus, dyspnea, nausea and vomiting, cyanosis, proteinuria and hematuria, jaundice, restlessness, and convulsion. Methemoglobinemia has been reported. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessaryBasic treatment: Establish a patent airway. Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with normal saline during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patent can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal ... . Cover skin burns with dry sterile dressings after decontamination ... . /Organic bases/Amines and related compounds/
5.Fire-fighting measures 5.1 Extinguishing media Suitable extinguishing mediaNot combustible. Extinguish fire using agent suitable for surrounding fire. Fight fire from protected location or maximum possible distance. Approach fire from upwind to avoid hazardous vapors and toxic decomposition products. Explosive decomposition may occur under fire conditions.
5.2 Specific hazards arising from the chemicalSpecial Hazards of Combustion Products: Nitrogen oxides - toxic fumes - react with water or steam to produce heat and corrosive liquids - can react violently with reducing materials. Behavior in Fire: May explode when exposed to heat or flame. Explodes at 129.44°C. (USCG, 1999)
5.3 Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures 6.1 Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautionsPersonal protection: particulate filter respirator adapted to the airborne concentration of the substance. Sweep spilled substance into covered sealable containers. If appropriate, moisten first to prevent dusting. Carefully collect remainder. Then store and dispose of according to local regulations.
6.3 Methods and materials for containment and cleaning upPick up and arrange disposal. Sweep up and shovel. Keep in suitable, closed containers for disposal.
7.Handling and storage 7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilitiesFireproof. Separated from incompatible materials. See Chemical Dangers. Cool. Dry. Well closed.Separate from oxidizing materials. store in a cool, dry, well-ventilated location. store away from heat, oxidizers, and sunlight. Outside or detached storage is preferred.
8.Exposure controls/personal protection 8.1 Control parameters Occupational Exposure limit valuesno data available
Biological limit valuesno data available
8.2 Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE) Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protectionWear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protectionWear dust mask when handling large quantities.
Thermal hazardsno data available
9.Physical and chemical properties| Physical state | white needles or flakes |
|---|---|
| Colour | Colorless cyrstals |
| Odour | no data available |
| Melting point/ freezing point | 7ºC |
| Boiling point or initial boiling point and boiling range | >100°C |
| Flammability | Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | 129.44°C (USCG, 1999) |
| Auto-ignition temperature | 129.44°C (USCG, 1999) |
| Decomposition temperature | <70°C |
| pH | no data available |
| Kinematic viscosity | no data available |
| Solubility | Very sol in water, liq ammonia, methanol; sparingly sol in ether, benzene, carbon disulfide, chloroform |
| Partition coefficient n-octanol/water (log value) | -1.5 |
| Vapour pressure | 9 mm Hg ( 40 °C) |
| Density and/or relative density | 1.078g/mLat 25°C |
| Relative vapour density | (air = 1): 1.1 |
| Particle characteristics | no data available |
no data available
10.2 Chemical stabilityUNSTABLE; RAPID DECOMP AT ROOM TEMP IN PRESENCE OF ATMOSPHERIC MOISTURE & CO2
10.3 Possibility of hazardous reactionsDangerous fire hazard when exposed to heat, flame, and oxidizers. May ignite spontaneously in air if a large surface area is exposed (e.g. precipitate on paper).HYDROXYLAMINE is a white solid, thermally unstable, decomposes rapidly at room temperature or when dissolved in hot water by internal oxidation-reduction. It should be stored below 10° C [Bailar, 1973, vol. 2, p. 272]. Explosive reaction with strong oxidizers (chromium trioxide, potassium dichromate) or powdered zinc upon heat. Reaction with zinc or calcium produces explosive bishydroxylamides. It ignites on contact with cupric sulfate, alkali metals (sodium, potassium), oxidants (e.g., barium oxide, barium peroxide, lead dioxide, potassium permanganate, chlorine), phosphorus trichloride and pentachloride. It reacts vigorously with hypochlorites, pyridine, carbonyls [Sax, 9th ed., 1996, p. 1875]. On contact with organic materials in thin layer (e.g., crystals on filter paper), it may ignite spontaneously in air. It explodes when heated above 70° C [Brauer, 1963, vol. 1, p. 502]. During a distillation process, an explosion occurred. Potassium hydroxide is thought to be involved in the explosion. Employees in the plant complained of chest pains and suffered chemical burns. Five people were killed by the explosion.
10.4 Conditions to avoidno data available
10.5 Incompatible materialsIncompatible with carbonyls; pyridine. Vigorous reaction with hypochlorites.
10.6 Hazardous decomposition productsWhen heated to decomposition it emits toxic fumes of NOx /nitrogen oxides/.
11.Toxicological information Acute toxicity- Oral: no data available
- Inhalation: no data available
- Dermal: no data available
no data available
Serious eye damage/irritationno data available
Respiratory or skin sensitizationno data available
Germ cell mutagenicityno data available
Carcinogenicityno data available
Reproductive toxicityno data available
STOT-single exposureno data available
STOT-repeated exposureno data available
Aspiration hazardno data available
12.Ecological information 12.1 Toxicity- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
no data available
12.3 Bioaccumulative potentialAn estimated BCF of 3 was calculated for hydroxylamine(SRC), using an estimated log Kow of -1.2(1,SRC) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
12.4 Mobility in soilUsing a structure estimation method based on molecular connectivity indices(1), the Koc for hydroxylamine can be estimated to be 14(SRC). According to a classification scheme(2), this estimated Koc value suggests that hydroxylamine is expected to have very high mobility in soil. A pKa of 5.94(3) indicates that hydroxylamine will partially exist in the protonated form in moist soils(SRC) and cations adsorb to soil stronger than neutral compounds.
12.5 Other adverse effectsno data available
13.Disposal considerations 13.1 Disposal methods ProductThe material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information 14.1 UN Number| ADR/RID: UN3082 | IMDG: UN3082 | IATA: UN3082 |
| ADR/RID: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S. |
| IMDG: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S. |
| IATA: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S. |
| ADR/RID: 9 | IMDG: 9 | IATA: 9 |
| ADR/RID: III | IMDG: III | IATA: III |
| ADR/RID: no | IMDG: no | IATA: no |
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Codeno data available
15.Regulatory information 15.1 Safety, health and environmental regulations specific for the product in question| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| hydroxylamine | hydroxylamine | 7803-49-8 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Not Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
| Creation Date | Aug 17, 2017 |
|---|---|
| Revision Date | Aug 17, 2017 |
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/