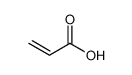
| Product name | acrylic acid |
|---|
| Product number | - |
|---|---|
| Other names | monoethylene carboxylic acid |
| Identified uses | For industry use only. Acrylic acid is used in the manufacture of plastics, in latex applications, in floor polish, in polymer solutions for coatings applications, emulsion polymers, paint formulations, leather finishings, and paper coatings. Acrylic acid is also used as a chemical intermediate. |
|---|---|
| Uses advised against | no data available |
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
Flammable liquids, Category 3
Acute toxicity - Oral, Category 4
Acute toxicity - Dermal, Category 4
Skin corrosion, Category 1A
Acute toxicity - Inhalation, Category 4
Hazardous to the aquatic environment, short-term (Acute) - Category Acute 1
2.2 GHS label elements, including precautionary statements| Pictogram(s) |  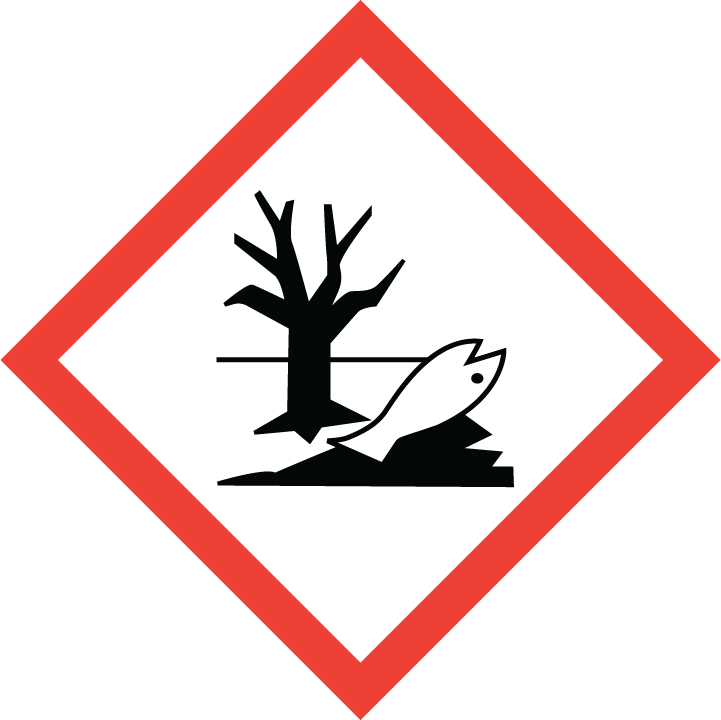 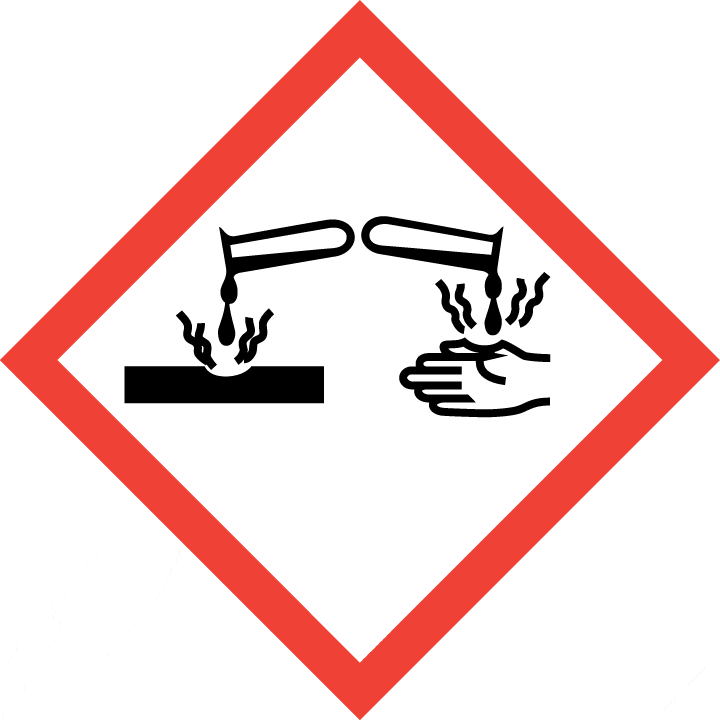  |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H226 Flammable liquid and vapour H302 Harmful if swallowed H312 Harmful in contact with skin H314 Causes severe skin burns and eye damage H332 Harmful if inhaled H400 Very toxic to aquatic life |
| Precautionary statement(s) | |
| Prevention | P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233 Keep container tightly closed. P240 Ground and bond container and receiving equipment. P241 Use explosion-proof [electrical/ventilating/lighting/...] equipment. P242 Use non-sparking tools. P243 Take action to prevent static discharges. P280 Wear protective gloves/protective clothing/eye protection/face protection. P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P261 Avoid breathing dust/fume/gas/mist/vapours/spray. P271 Use only outdoors or in a well-ventilated area. P273 Avoid release to the environment. |
| Response | P303+P361+P353 IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water [or shower]. P370+P378 In case of fire: Use ... to extinguish. P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor/…if you feel unwell. P330 Rinse mouth. P302+P352 IF ON SKIN: Wash with plenty of water/... P312 Call a POISON CENTER/doctor/…if you feel unwell. P321 Specific treatment (see ... on this label). P362+P364 Take off contaminated clothing and wash it before reuse. P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 Wash contaminated clothing before reuse. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P310 Immediately call a POISON CENTER/doctor/… P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P391 Collect spillage. |
| Storage | P403+P235 Store in a well-ventilated place. Keep cool. P405 Store locked up. |
| Disposal | P501 Dispose of contents/container to ... |
none
3.Composition/information on ingredients 3.1 Substances| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| acrylic acid | acrylic acid | 79-10-7 | none | 100% |
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaledFresh air, rest. Half-upright position. Refer for medical attention.
In case of skin contactRemove contaminated clothes. Rinse skin with plenty of water or shower. Refer for medical attention .
In case of eye contactFirst rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowedRinse mouth. Do NOT induce vomiting. Refer immediately for medical attention.
4.2 Most important symptoms/effects, acute and delayedMay burn skin or eyes upon short contact. INHALATION: eye and nasal irritation and lacrimation. INGESTION: may cause severe damage to the gastrointestinal tract. (USCG, 1999)
Exposure Routes: inhalation, skin absorption, ingestion, skin and/or eye contact Symptoms: Irritation eyes, skin, respiratory system; eye, skin burns; skin sensitization Target Organs: Eyes, skin, respiratory system (NIOSH, 2016)
4.3 Indication of immediate medical attention and special treatment needed, if necessaryImmediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand-valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR as necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Organic acids and related compounds/
5.Fire-fighting measures 5.1 Extinguishing media Suitable extinguishing mediaUse dry chemical, carbon dioxide, or alcohol foam extinguishers. Vapors are heavier than air and will collect in low areas. Vapors may travel long distances to ignition sources and flashback. Vapors in confined areas may explode when exposed to fire. Storage containers and parts of containers may rocket great distances, in many directions. In advanced or massive fires, fire fighting should be done from a safe distance or from a protected location. If a leak or spill has not ignited, use water spray to disperse the vapors. Water spray may be used to flush spills away from exposures and to dilute spills to nonflammable mixtures. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Notify local health and fire officials and pollution control agencies. From a secure, explosion-proof location, use water spray to cool exposed containers. If cooling streams are ineffective (venting sound increases in volume and pitch, tank discolors or shows any signs of deforming), withdraw immediately to a secure position.
5.2 Specific hazards arising from the chemicalSpecial Hazards of Combustion Products: Toxic vapors are generated when heated Behavior in Fire: May polymerize and explode (USCG, 1999)
Excerpt from ERG Guide 132P [Flammable Liquids - Corrosive]: Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Those substances designated with a (P) may polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. (ERG, 2016)
5.3 Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures 6.1 Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautionsEvacuate danger area! Consult an expert! Personal protection: complete protective clothing including self-contained breathing apparatus. Ventilation. Do NOT let this chemical enter the environment. Collect leaking liquid in sealable containers. Absorb remaining liquid in sand or inert absorbent. Then store and dispose of according to local regulations.
6.3 Methods and materials for containment and cleaning upSRP: Wastewater from contaminant suppression, cleaning of protective clothing/equipment, or contaminated sites should be contained and evaluated for subject chemical or decomposition product concentrations. Concentrations shall be lower than applicable environmental discharge or disposal criteria. Alternatively, pretreatment and/or discharge to a permitted wastewater treatment facility is acceptable only after review by the governing authority and assurance that "pass through" violations will not occur. Due consideration shall be given to remediation worker exposure (inhalation, dermal and ingestion) as well as fate during treatment, transfer and disposal. If it is not practicable to manage the chemical in this fashion, it must be evaluated in accordance with EPA 40 CFR Part 261, specifically Subpart B, in order to determine the appropriate local, state and federal requirements for disposal.
7.Handling and storage 7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilitiesFireproof. Separated from strong oxidants, strong bases, strong acids and food and feedstuffs. Keep in the dark. Store only if stabilized. Store in an area without drain or sewer access. Storage conditions may vary according to the type of inhibitor used. Refer to the manufacturer's instructions for proper storage conditions. See Notes.Acrylic acid should be stored in a detached, cool, well-ventilated, non-combustible place, and its containers should be protected against physical damage. Acrylic acid can be stored only in vessels lined with glass, stainless steel, aluminum, or polyethylene. In order to inhibit polymerization during transport and storage, 200 ppm MeHQ (the monomethyl ether of hydroquinone) is commonly added to acrylic acid by the manufacturer. The presence of oxygen is required for the inhibitor to be effective. A major concern during the storage of acrylic acid is the avoidance of elevated temperatures as well as freezing, since both can lead to a failure of the inhibitor system. Ideally acrylic acid should be stored within a temperature range of 15 to 25°C.
8.Exposure controls/personal protection 8.1 Control parameters Occupational Exposure limit valuesRecommended Exposure Limit: 10-hour Time-Weighted Average: 2 ppm (6 mg/cu m) [skin].
Biological limit valuesno data available
8.2 Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE) Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protectionWear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protectionWear dust mask when handling large quantities.
Thermal hazardsno data available
9.Physical and chemical properties| Physical state | Colorless liquid |
|---|---|
| Colour | Volatile liquid |
| Odour | Acrid odor and fumes |
| Melting point/ freezing point | 315°C(lit.) |
| Boiling point or initial boiling point and boiling range | 139°C(lit.) |
| Flammability | Class II Combustible Liquid: Fl.P. at or above 37.78°C and below 60°C.Flammable. Many reactions may cause fire or explosion. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | Lower flammable limit: 2.4% by volume; Upper flammable limit: 8.0% by volume |
| Flash point | 46°C |
| Auto-ignition temperature | 395.56°C |
| Decomposition temperature | no data available |
| pH | pH = 3 (approximately) |
| Kinematic viscosity | no data available |
| Solubility | In water:MISCIBLE |
| Partition coefficient n-octanol/water (log value) | no data available |
| Vapour pressure | 4 mm Hg ( 20 °C) |
| Density and/or relative density | 1.051g/mLat 25°C(lit.) |
| Relative vapour density | 2.5 (vs air) |
| Particle characteristics | no data available |
no data available
10.2 Chemical stabilityAcrylic acid and methacrylic acid readily polymerize in the presence of light, heat and oxygen, and also under the action of oxidizing agents such as peroxides.
10.3 Possibility of hazardous reactionsFlammable liquid. ... A fire hazard when exposed to heat or flame.Vapours are uninhibited and may polymerize in vents or flame arresters, causing blockage.Dust explosion possible if in powder or granular form, mixed with air. If dry, it can be charged electrostatically by swirling, pneumatic transport, pouring, etc.ACRYLIC ACID may polymerize violently especially when the frozen acid is partially thawed (freezing point 12°C or 11.67°C). Frozen acid should be melted at room temperature and the process should be well stirred. Do not use heat during the melting process [Kirk-Othmer, 3rd ed., Vol. 1, 1978, p. 330]. Corrodes iron and steel and polymerization may occur on contact with iron salts. The uninhibited acid polymerizes exothermically at ambient temperature and explodes if confined. The inhibitor (usually hydroquinone) greatly reduces the tendency to polymerize. Explosive polymerization can also occur with strong bases, amines, ammonia, oleum, chlorosulfonic acid, and peroxides. Mixing with 2-aminoethanol, 28% ammonium hydroxide, ethylenediamine or ethyleneimine in a closed container causes an increase in temperature and pressure. Can react violently with oxidizing reagents and strong bases [Bretherick, 5th ed., 1995, p. 419].
10.4 Conditions to avoidno data available
10.5 Incompatible materialsViolent reaction with strong oxidizers. Incompatible with sulfuric acid, caustics, ammonia, amines, isocyanates, alkylene oxides, epichlorohydrin, toluene diamine, oleum, pyridine, methyl pyridine, n-methyl pyrrolidone, 2-methyl-6-ether aniline, aniline, ethylene diamine, ethyleneimine, and 2-aminoethanol. Severely corrodes carbon steel and iron; attacks other metals. May accumulate static electrical charges and may cause ignition of its vapors.
10.6 Hazardous decomposition productsAcrylic acid rapidly decomposes in the atmosphere by photochemical attack on the double bond.
11.Toxicological information Acute toxicity- Oral: LD50 Rat oral 193 mg/kg
- Inhalation: LC50 Rat inhalation 1200 ppm/4 hr
- Dermal: LD50 Rabbit percutaneous 290 mg/kg
no data available
Serious eye damage/irritationno data available
Respiratory or skin sensitizationno data available
Germ cell mutagenicityno data available
CarcinogenicityEvaluation: No epidemiological data relevant to the carcinogenicity of acrylic acid were available. No experimental data relevant to the carcinogenicity of acrylic acid were available. Overall evaluation: Acrylic acid is not classifiable as to its carcinogenicity in humans (Group 3).
Reproductive toxicityNo information is available on the reproductive or developmental effects of acrylic acid in humans. Decreased body weight gain and decreased fertility were reported in one study of rats exposed to acrylic acid by ingestion, although the decrease in fertility was not statistically significant compared with the control. Embryotoxic and teratogenic effects (birth defects) were observed in rats injected with acrylic acid.
STOT-single exposureno data available
STOT-repeated exposureno data available
Aspiration hazardno data available
12.Ecological information 12.1 Toxicity- Toxicity to fish: LC50; Species: Brachydanio rerio (Zebra fish); Conditions: semi-static, open system, measured concentration; Concentration: 222 mg/L/96 hr
- Toxicity to daphnia and other aquatic invertebrates: LC50; Species: Daphnia magna (Water flea, age < or =24 hr); Conditions: freshwater, static, 20-22°C, pH 7.6-7.7; Concentration: 270 mg/L for 24 hr /formulation
- Toxicity to algae: Toxicity threshold (cell multiplication inhibition test): Algae (Microcystis aeruginosa) 0.15 mg/L.
- Toxicity to microorganisms: no data available
AEROBIC: Acrylic acid, present at 100 mg/L, reached 68% of its theoretical BOD in 2 weeks using an activated sludge inoculum at 30 mg/L and the Japanese MITI test(1). The BOD5/COD ratio for acrylic acid was determined to be 0.22, which is indicative of significant potential for biodegradability(2). A microbial degradation study of acrylic acid in soil indicated that acrylic acid, formed from hydrolysis of acrylamide added to soil, was totally degraded within 15 days of its formation(3).
12.3 Bioaccumulative potentialAn estimated BCF of 3 was calculated in fish for acrylic acid(SRC), using a log Kow of 0.35(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
12.4 Mobility in soilKoc values for acrylic acid have been reported as 6 in Washington clay/loam (29% sand, 42% silt, 29% clay, 3.39% organic carbon, pH 6.0), 9 in Canfield loam (45% sand, 42% silt, 13% clay, 4.58% organic carbon, pH 6.1), 29 in Ellsworth loam (35% sand, 40% silt, 25% clay, 1.42% organic carbon, pH 7.2), 137 in Tyner loamy sand (79% sand, 14% silt, 7% clay, 0.46% organic carbon, pH 5.2), and 33 in sandy loam sediment (53% sand, 28% silt, 19% clay, 1.23% organic carbon, pH 7.5)(1) According to a classification scheme(2), these Koc values suggest that acrylic acid is expected to have very high to high mobility in soil. The pKa of acrylic acid is 4.26(3), indicating that this compound will exist almost entirely in anion form in the environment and anions generally do not adsorb more strongly to soils containing organic carbon and clay than their neutral counterparts(4).
12.5 Other adverse effectsno data available
13.Disposal considerations 13.1 Disposal methods ProductThe material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information 14.1 UN Number| ADR/RID: UN2218 | IMDG: UN2218 | IATA: UN2218 |
| ADR/RID: ACRYLIC ACID, STABILIZED |
| IMDG: ACRYLIC ACID, STABILIZED |
| IATA: ACRYLIC ACID, STABILIZED |
| ADR/RID: 3 | IMDG: 3 | IATA: 3 |
| ADR/RID: II | IMDG: II | IATA: II |
| ADR/RID: yes | IMDG: yes | IATA: yes |
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Codeno data available
15.Regulatory information 15.1 Safety, health and environmental regulations specific for the product in question| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| acrylic acid | acrylic acid | 79-10-7 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
| Creation Date | Aug 12, 2017 |
|---|---|
| Revision Date | Aug 12, 2017 |
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/