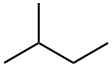
| Product name | isobutane |
|---|
| Product number | - |
|---|---|
| Other names | Propane, 2-methyl- |
| Identified uses | For industry use only. Food additives |
|---|---|
| Uses advised against | no data available |
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
no data available
2.2 GHS label elements, including precautionary statements| Pictogram(s) | no data available |
|---|---|
| Signal word | no data available |
| Hazard statement(s) | no data available |
| Precautionary statement(s) | |
| Prevention | no data available |
| Response | no data available |
| Storage | no data available |
| Disposal | no data available |
no data available
3.Composition/information on ingredients 3.1 Substances| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| isobutane | isobutane | 75-28-5 | none | 100% |
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaledFresh air, rest. Refer for medical attention.
In case of skin contactON FROSTBITE: rinse with plenty of water, do NOT remove clothes. Refer for medical attention .
In case of eye contactFirst rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowedNever give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
4.2 Most important symptoms/effects, acute and delayedCentral nervous system depression ranging from dizziness and incoordination to anesthesia and respiratory arrest, depending on concentration and extent of inhalation. Irregular heartbeat is rare but is a dangerous complication at anesthetic levels. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessaryINHALATION: Fresh air, rest. Refer for medical attention. Half-upright position. Artificial respiration may be needed. Refer for medical attention. SKIN: ON FROSTBITE: rinse with plenty of water, do NOT remove clothes. Refer for medical attention. EYES: First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then take to a doctor.
5.Fire-fighting measures 5.1 Extinguishing media Suitable extinguishing mediaButane is a flammable gas. In case of fire, stop the flow of gas if it can be done safely. Use dry chemical, carbon dioxide, or halon extinguishers. Use water to keep fire-exposed containers cool and to protect personnel doing the shut-off. If leak or spill has caught fire, use water spray to disperse gas and to protect personnel shutting off leak. If cooling streams are ineffective (venting sound increases in volume and pitch, tank discolors, or shows any signs of deforming), withdraw immediately to a secure position. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Contact your Department of Environmental Protection or your regional office of the federal EPA for specific recommendations. If employees are required to fight fires, they must be properly trained and equipped. OSHA 1910.156. /Butanes/
5.2 Specific hazards arising from the chemicalExcerpt from ERG Guide 115 [Gases - Flammable (Including Refrigerated Liquids)]: EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. (ERG, 2016)
5.3 Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures 6.1 Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautionsEvacuate danger area! Consult an expert! Ventilation. Remove all ignition sources. NEVER direct water jet on liquid. Personal protection: filter respirator for organic vapours of low boiling point adapted to the airborne concentration of the substance.
6.3 Methods and materials for containment and cleaning upEvacuate and restrict persons not wearing protective equipment from area of spill or leak until cleanup is complete. Remove all ignition sources. Keep the gas concentration below the explosive limit range by forced ventilation. Stop the flow of gas. If source of leak is a cylinder and the leak cannot be stopped in place, remove leaking cylinder to a safe place in the open air, and repair leak or allow cylinder to dissipate to the atmosphere. If employees are required to clean up spills, they must be properly trained and equipped. OSHA 1910.120(q) may be applicable. /Butanes/
7.Handling and storage 7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilitiesFireproof. Cool.Store in tightly closed containers in a cool, well-ventilated area away from incompatible materials ... and heat. /Butanes/
8.Exposure controls/personal protection 8.1 Control parameters Occupational Exposure limit valuesRecommended Exposure Limit: 10 Hr Time-Weighted Avg: 800 ppm (1900 mg/cu m).
Biological limit valuesno data available
8.2 Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE) Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protectionWear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protectionWear dust mask when handling large quantities.
Thermal hazardsno data available
9.Physical and chemical properties| Physical state | colourless odourless gas (or colourless cryogenic liquid)under pressure |
|---|---|
| Colour | Colorless gas [Note: Shipped as a liquified compressed gas. A liquid below 11deg F] |
| Odour | Gasoline-like or natural gas odor. |
| Melting point/ freezing point | -160ºC(lit.) |
| Boiling point or initial boiling point and boiling range | ?12°C(lit.) |
| Flammability | Flammable GasExtremely flammable. |
| Lower and upper explosion limit / flammability limit | Lower flammable limit: 1.8% by volume; Upper flammable limit: 8.4% by volume |
| Flash point | -83°C |
| Auto-ignition temperature | 460°C |
| Decomposition temperature | no data available |
| pH | no data available |
| Kinematic viscosity | 0.238 cP at -10°C |
| Solubility | Slight (NIOSH, 2016) |
| Partition coefficient n-octanol/water (log value) | log Kow = 2.76 |
| Vapour pressure | 72.2 psi ( 37.7 °C) |
| Density and/or relative density | 2.064g/mLat 25°C(lit.) |
| Relative vapour density | 2.01 (21 °C, vs air) |
| Particle characteristics | no data available |
no data available
10.2 Chemical stabilityStable under recommended storage conditions.
10.3 Possibility of hazardous reactionsSEVERE, WHEN EXPOSED TO HEAT OR FLAME.The gas is heavier than air and may travel along the ground; distant ignition possible. As a result of flow, agitation, etc., electrostatic charges can be generated.ISOBUTANE is incompatible with the following: Strong oxidizers (e.g., nitrates & perchlorates), chlorine, fluorine, (nickel carbonyl + oxygen) (NIOSH, 2016).
10.4 Conditions to avoidno data available
10.5 Incompatible materialsReacts with strong oxidants, acetylene, halogens and nitrogen oxides causing fire and explosion hazard.
10.6 Hazardous decomposition productsno data available
11.Toxicological information Acute toxicity- Oral: no data available
- Inhalation: LC50 Rat inhalation (15 min) 570000 ppm
- Dermal: no data available
no data available
Serious eye damage/irritationno data available
Respiratory or skin sensitizationno data available
Germ cell mutagenicityno data available
Carcinogenicityno data available
Reproductive toxicityno data available
STOT-single exposureno data available
STOT-repeated exposureno data available
Aspiration hazardno data available
12.Ecological information 12.1 Toxicity- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
AEROBIC: Indigenous soil microorganisms will biodegrade petroleum products under aerobic conditions(1). Biodegradation may occur under anaerobic conditions at a much slower rate, particularly by sulfur-reducing bacteria(2). It was demonstrated that isobutane is oxidized by bacteria as the sole source of carbon and energy(1). The bacteria were isolated from lake water and soil near a refinery in New Jersey. In the case of a Brevibacterium sp., the rate of oxidation of isobutane was about 70% that of n-butane. However, no isobutane degraded in 8 days when incubated with groundwater contaminated with gasoline(3). Isobutane was subject to biodegradation in a microcosm designed to simulate conditions in Narragansett Bay, RI in September and November(4). The biodegradation half-lives for isobutane were 16-26 days for September (20°C) and 33-139 days for November (10°C)(4). The degradation rate was slower than for n-butane and fell between that of propane and ethane. The biodegradation increased markedly on acclimation. Approximately 95% of the isobutane present in the microcosm after 12.9 days degraded within the next 2 days(1).
12.3 Bioaccumulative potentialAn estimated BCF of 27 was calculated for isobutane(SRC), using a log Kow of 2.76(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
12.4 Mobility in soilUsing an estimation method based on molecular connectivity indices(1), the Koc for isobutane is estimated to be 35(SRC). According to a suggested classification scheme(2), this Koc value suggests that isobutane will have very high mobility in soil(SRC).
12.5 Other adverse effectsno data available
13.Disposal considerations 13.1 Disposal methods ProductThe material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information 14.1 UN Number| ADR/RID: UN1969 | IMDG: UN1969 | IATA: UN1969 |
| ADR/RID: ISOBUTANE |
| IMDG: ISOBUTANE |
| IATA: ISOBUTANE |
| ADR/RID: 2.1 | IMDG: 2.1 | IATA: 2.1 |
| ADR/RID: unknown | IMDG: unknown | IATA: unknown |
| ADR/RID: no | IMDG: no | IATA: no |
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Codeno data available
15.Regulatory information 15.1 Safety, health and environmental regulations specific for the product in question| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| isobutane | isobutane | 75-28-5 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
| Creation Date | Aug 16, 2017 |
|---|---|
| Revision Date | Aug 16, 2017 |
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/